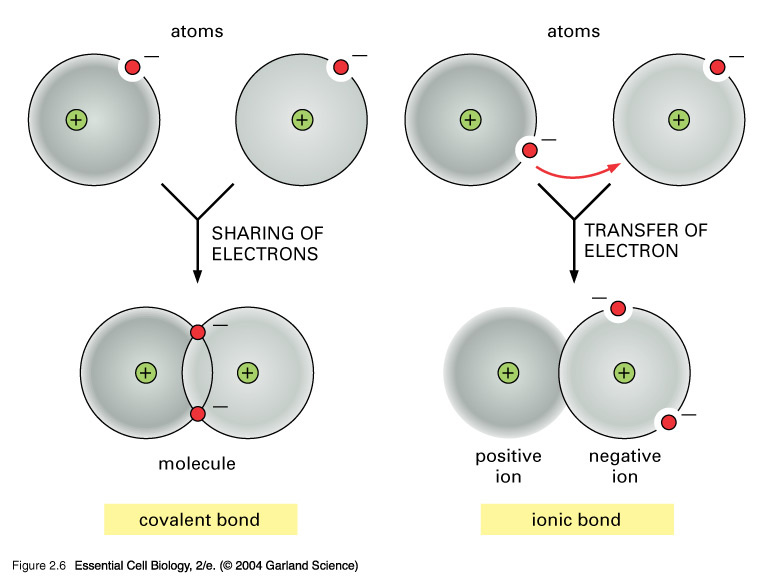
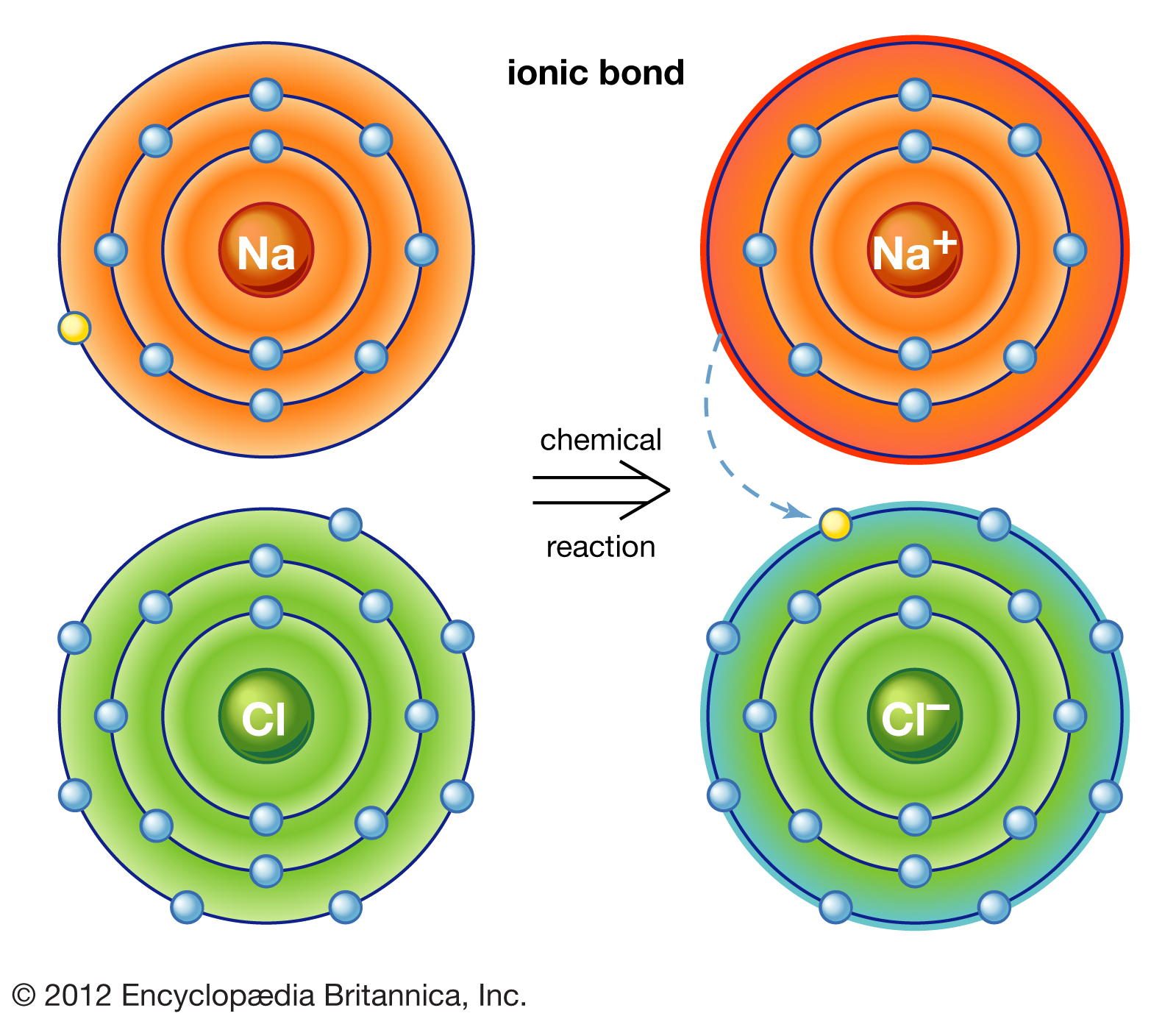
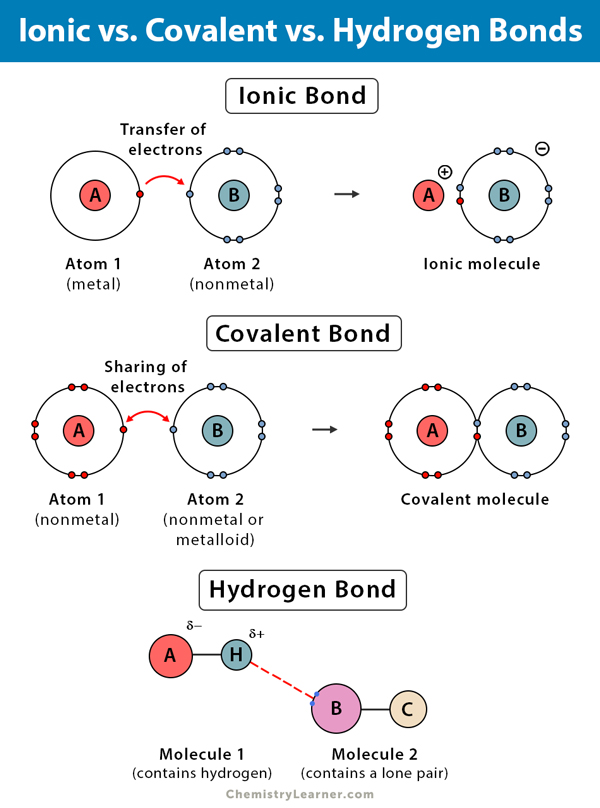
Why Do Atoms Form Ionic And Covalent Bonds - Ionic bonds require at least one electron. Charged atoms are called ions. In ionic bonding, atoms transfer electrons to each other. Because opposite charges attract (while like charges repel), these.
In ionic bonding, atoms transfer electrons to each other. Ionic bonds require at least one electron. Because opposite charges attract (while like charges repel), these. Charged atoms are called ions.
Charged atoms are called ions. Because opposite charges attract (while like charges repel), these. In ionic bonding, atoms transfer electrons to each other. Ionic bonds require at least one electron.
What is the difference between covalent and ionic bonding? Socratic
Because opposite charges attract (while like charges repel), these. In ionic bonding, atoms transfer electrons to each other. Charged atoms are called ions. Ionic bonds require at least one electron.
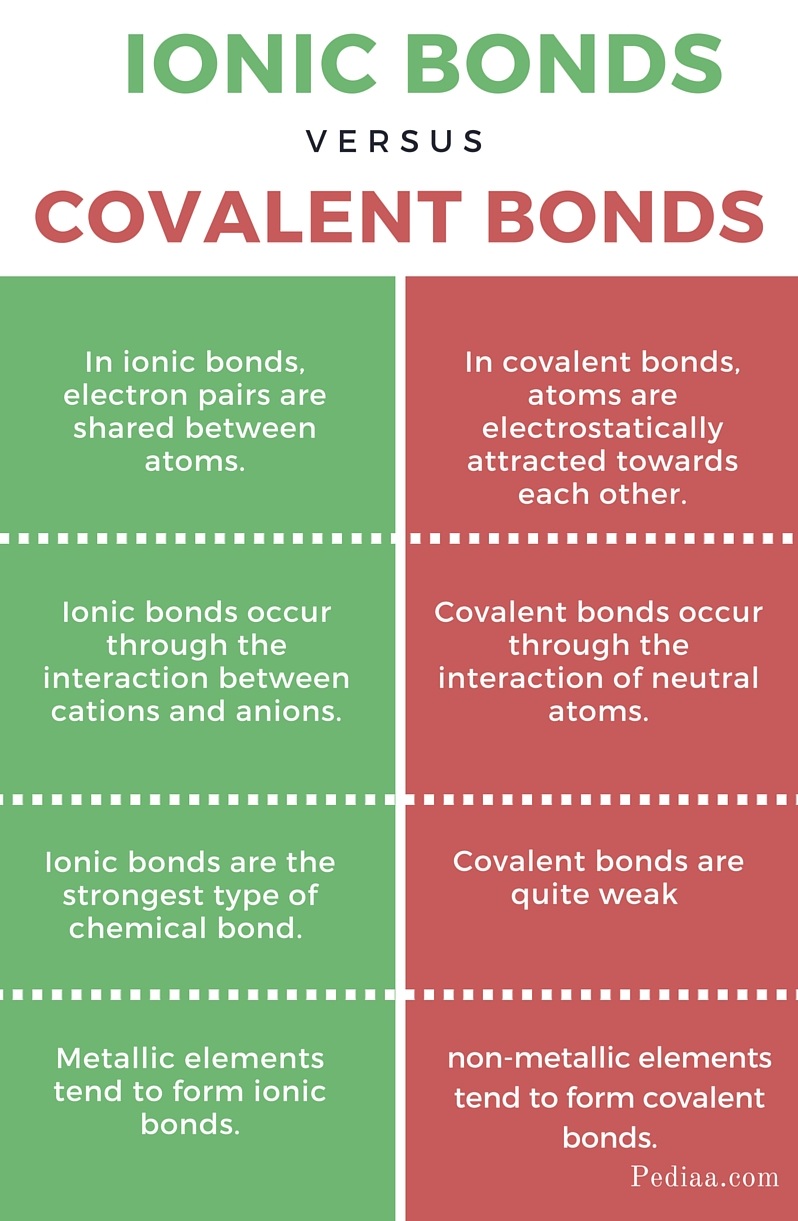
Difference Between Covalent and Ionic Bonds
Charged atoms are called ions. In ionic bonding, atoms transfer electrons to each other. Because opposite charges attract (while like charges repel), these. Ionic bonds require at least one electron.
Covalent bond Definition, Properties, Examples, & Facts Britannica
Ionic bonds require at least one electron. In ionic bonding, atoms transfer electrons to each other. Charged atoms are called ions. Because opposite charges attract (while like charges repel), these.
How do atoms form covalent bond?
Charged atoms are called ions. Because opposite charges attract (while like charges repel), these. Ionic bonds require at least one electron. In ionic bonding, atoms transfer electrons to each other.
How To Form Ionic Bonds
Ionic bonds require at least one electron. Charged atoms are called ions. In ionic bonding, atoms transfer electrons to each other. Because opposite charges attract (while like charges repel), these.
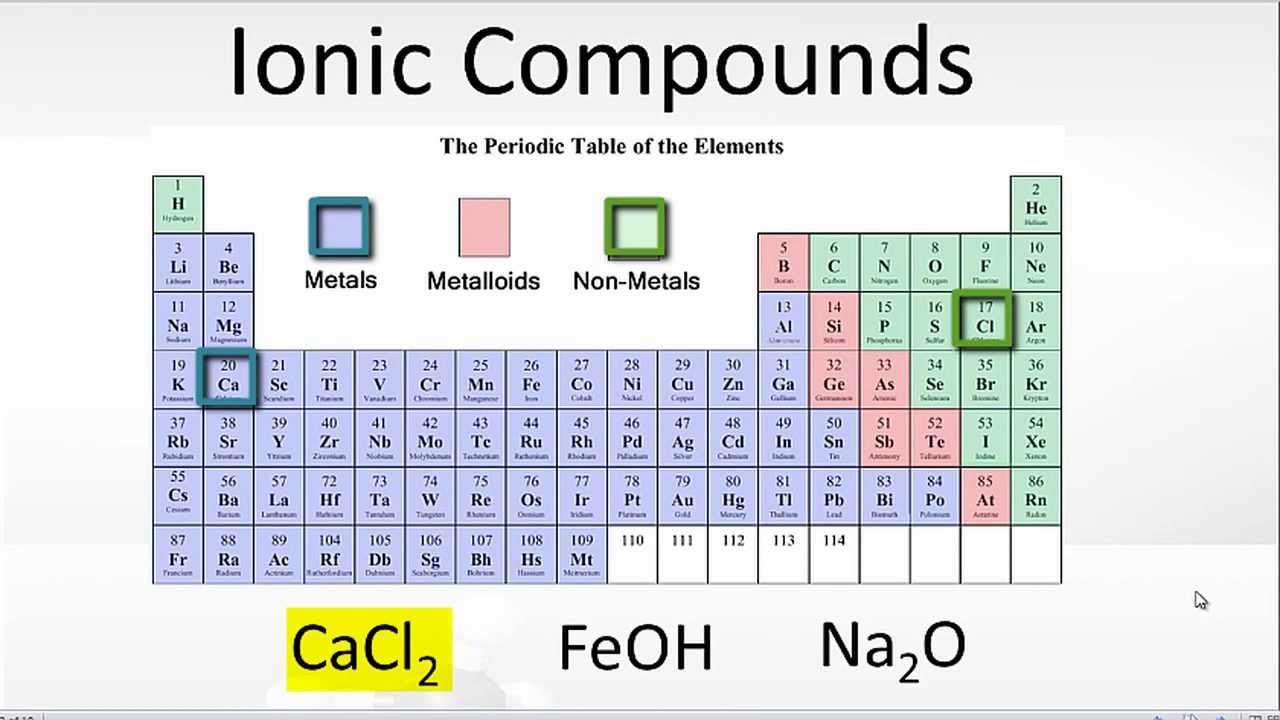
Compounds With Both Ionic and Covalent Bonds
Because opposite charges attract (while like charges repel), these. Ionic bonds require at least one electron. Charged atoms are called ions. In ionic bonding, atoms transfer electrons to each other.
Reading Covalent Bonds Biology I
In ionic bonding, atoms transfer electrons to each other. Ionic bonds require at least one electron. Charged atoms are called ions. Because opposite charges attract (while like charges repel), these.
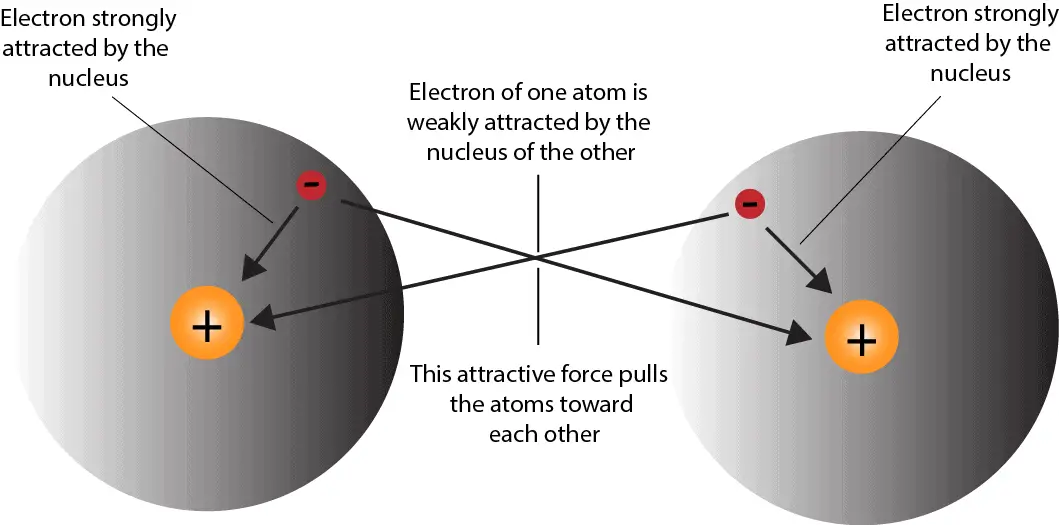
Chapter 5.6 Properties of Polar Covalent Bonds Chemistry LibreTexts
Because opposite charges attract (while like charges repel), these. Charged atoms are called ions. Ionic bonds require at least one electron. In ionic bonding, atoms transfer electrons to each other.
chemistry knowledge Comparison between Covalent and Ionic Bond
Ionic bonds require at least one electron. Charged atoms are called ions. Because opposite charges attract (while like charges repel), these. In ionic bonding, atoms transfer electrons to each other.
Charged Atoms Are Called Ions.
Ionic bonds require at least one electron. Because opposite charges attract (while like charges repel), these. In ionic bonding, atoms transfer electrons to each other.