Case Report Form - Case report form (crf) is a specialized document in clinical research. Sop 350 designing and developing a case report form. We report an atypical pediatric case of tuberculosis with lymphadenopathy. A standard operating procedure for the preparation and maintenance of a trial master file using. Learn how to create standardized and reliable case report forms (crfs) for clinical trials. It should be study protocol.
Learn how to create standardized and reliable case report forms (crfs) for clinical trials. Sop 350 designing and developing a case report form. Case report form (crf) is a specialized document in clinical research. A standard operating procedure for the preparation and maintenance of a trial master file using. It should be study protocol. We report an atypical pediatric case of tuberculosis with lymphadenopathy.
Learn how to create standardized and reliable case report forms (crfs) for clinical trials. A standard operating procedure for the preparation and maintenance of a trial master file using. Sop 350 designing and developing a case report form. We report an atypical pediatric case of tuberculosis with lymphadenopathy. Case report form (crf) is a specialized document in clinical research. It should be study protocol.
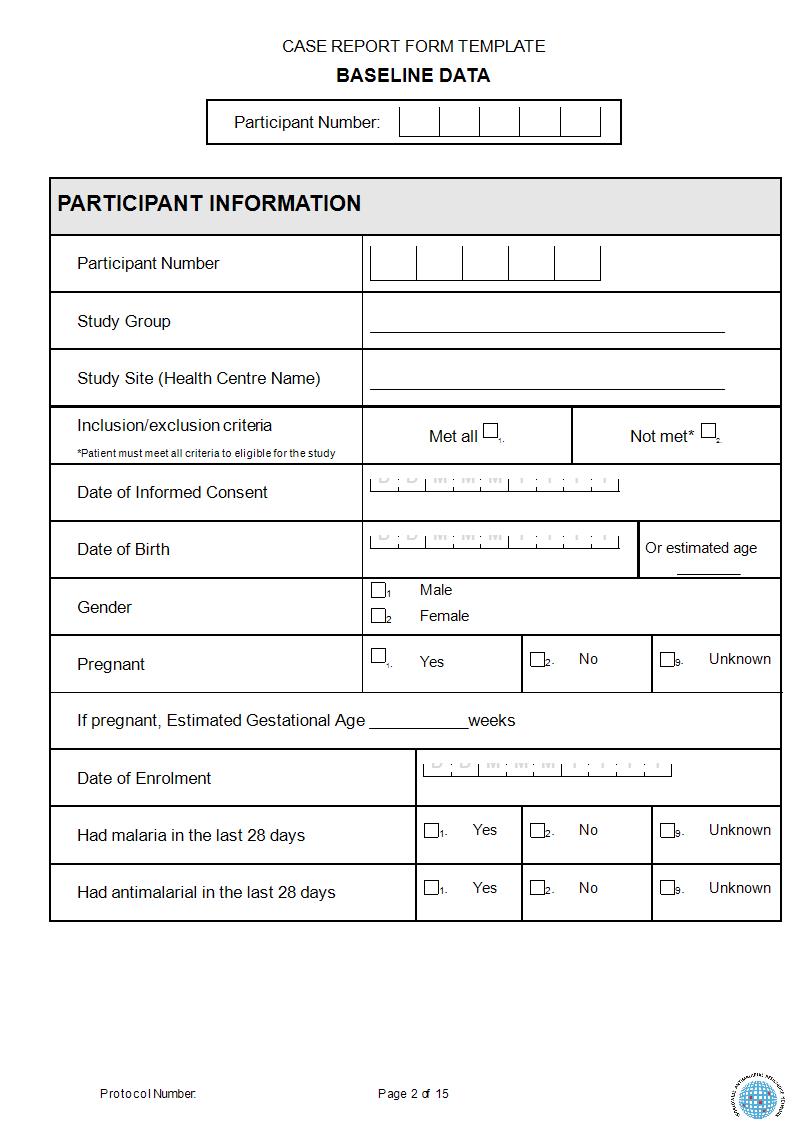
Case Report Form Template
A standard operating procedure for the preparation and maintenance of a trial master file using. It should be study protocol. Learn how to create standardized and reliable case report forms (crfs) for clinical trials. Case report form (crf) is a specialized document in clinical research. Sop 350 designing and developing a case report form.
Blank Case Report Form RIAT Support Center
We report an atypical pediatric case of tuberculosis with lymphadenopathy. Learn how to create standardized and reliable case report forms (crfs) for clinical trials. Case report form (crf) is a specialized document in clinical research. Sop 350 designing and developing a case report form. A standard operating procedure for the preparation and maintenance of a trial master file using.
Case Report Form Template
Learn how to create standardized and reliable case report forms (crfs) for clinical trials. We report an atypical pediatric case of tuberculosis with lymphadenopathy. Sop 350 designing and developing a case report form. Case report form (crf) is a specialized document in clinical research. It should be study protocol.
What Is a Case Report Form? [ Importance, Tips, Samples ]
It should be study protocol. Sop 350 designing and developing a case report form. A standard operating procedure for the preparation and maintenance of a trial master file using. Learn how to create standardized and reliable case report forms (crfs) for clinical trials. We report an atypical pediatric case of tuberculosis with lymphadenopathy.
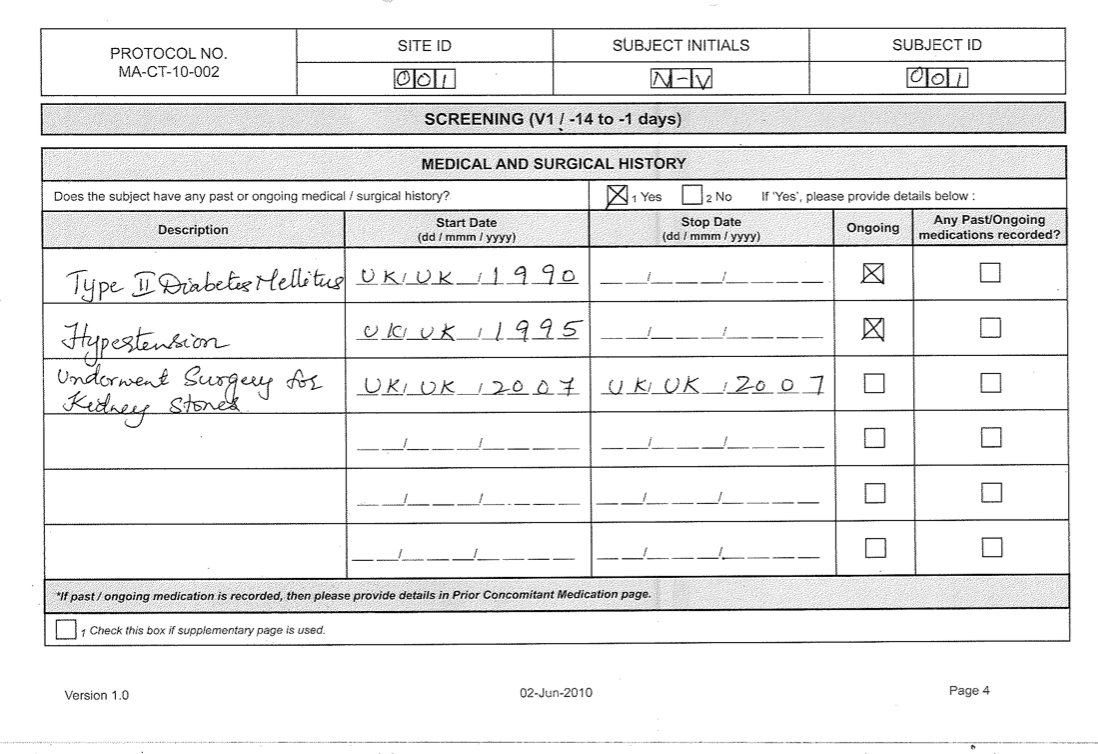
Case Report Form Template Clinical Trials
Sop 350 designing and developing a case report form. Learn how to create standardized and reliable case report forms (crfs) for clinical trials. A standard operating procedure for the preparation and maintenance of a trial master file using. We report an atypical pediatric case of tuberculosis with lymphadenopathy. It should be study protocol.
Free 15+ Case Report Forms In Pdf Ms Word inside Case Report Form
We report an atypical pediatric case of tuberculosis with lymphadenopathy. A standard operating procedure for the preparation and maintenance of a trial master file using. It should be study protocol. Learn how to create standardized and reliable case report forms (crfs) for clinical trials. Sop 350 designing and developing a case report form.
Case Report Form Template
Learn how to create standardized and reliable case report forms (crfs) for clinical trials. A standard operating procedure for the preparation and maintenance of a trial master file using. We report an atypical pediatric case of tuberculosis with lymphadenopathy. It should be study protocol. Case report form (crf) is a specialized document in clinical research.
Case Report Form Template Download in Word, Google Docs, Apple Pages
We report an atypical pediatric case of tuberculosis with lymphadenopathy. It should be study protocol. Learn how to create standardized and reliable case report forms (crfs) for clinical trials. A standard operating procedure for the preparation and maintenance of a trial master file using. Sop 350 designing and developing a case report form.
Case Report Form RIAT Support Center
Case report form (crf) is a specialized document in clinical research. We report an atypical pediatric case of tuberculosis with lymphadenopathy. Learn how to create standardized and reliable case report forms (crfs) for clinical trials. It should be study protocol. A standard operating procedure for the preparation and maintenance of a trial master file using.
FREE 15+ Case Report Forms in PDF MS Word
Sop 350 designing and developing a case report form. Learn how to create standardized and reliable case report forms (crfs) for clinical trials. It should be study protocol. A standard operating procedure for the preparation and maintenance of a trial master file using. Case report form (crf) is a specialized document in clinical research.
Case Report Form (Crf) Is A Specialized Document In Clinical Research.
A standard operating procedure for the preparation and maintenance of a trial master file using. It should be study protocol. Sop 350 designing and developing a case report form. We report an atypical pediatric case of tuberculosis with lymphadenopathy.
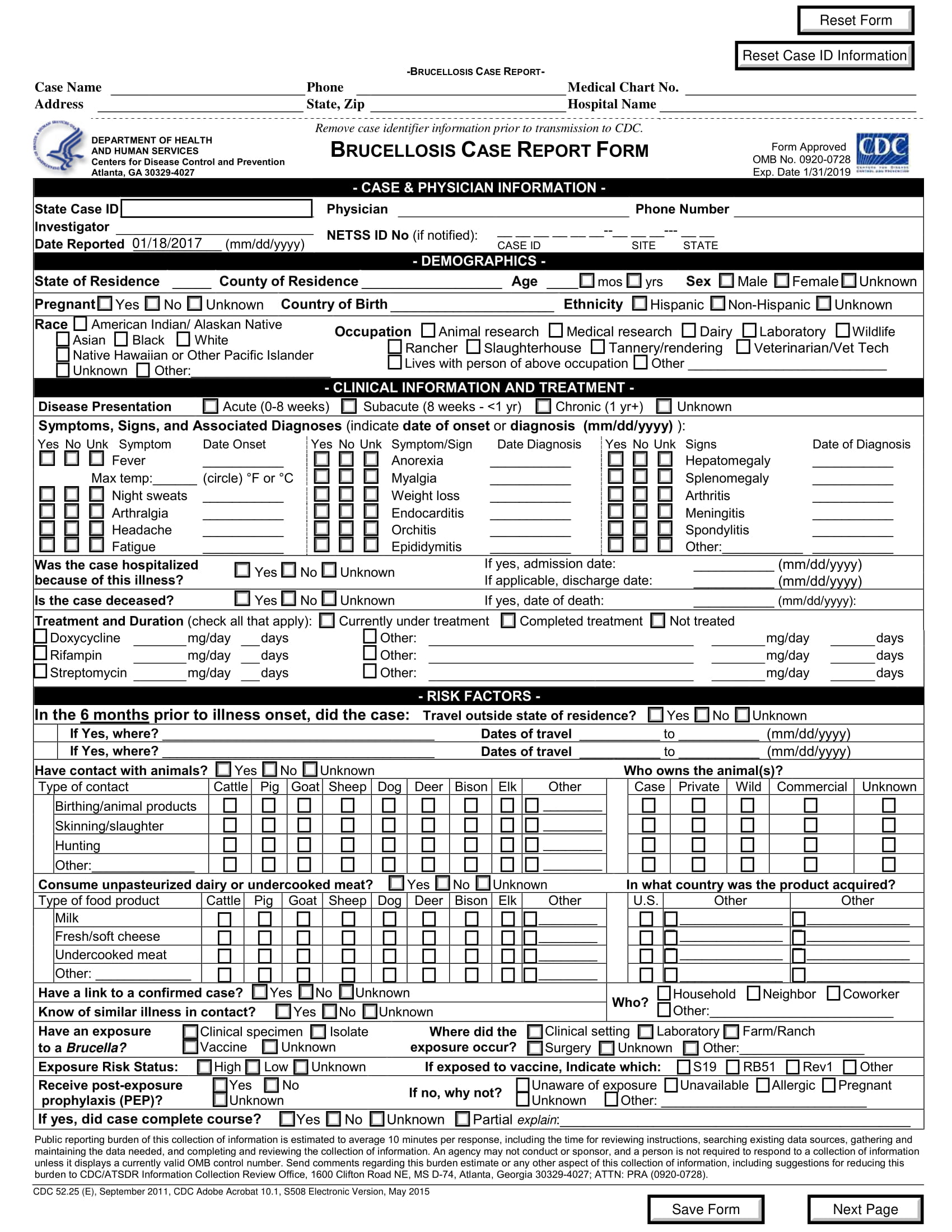


![What Is a Case Report Form? [ Importance, Tips, Samples ]](https://images.sampleforms.com/wp-content/uploads/2018/02/Syndrome-Case-Report-Form-1.jpg)